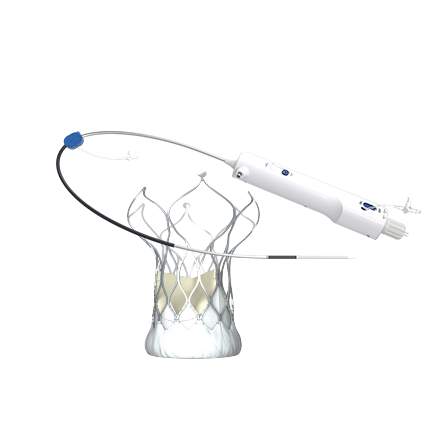
Seoul and Gwangju, South Korea—On June 11-12, 2025, the VitaFlow Liberty® Transcatheter Aortic Valve and Retrievable Delivery System (hereinafter referred to as "VitaFlow Liberty®"), independently developed by MicroPort CardioFlow Medtech Corporation (CardioFlow, stock code: 02160.HK), successfully completed South Korea's first five commercial implants at two leading medical centers - Seoul St. Mary's Hospital (The Catholic University of Korea) and Chonnam National University Hospital.
The procedures were supervised by Professor Zhang Junjie, Vice President of Nanjing First Hospital, as the mentoring expert, with Dr. Chang Ki Yuk from Seoul St. Mary's Hospital and Dr. Kim Juhan from Chonnam National University Hospital serving as the primary operators. Postoperative evaluations showed no significant paravalvular leakage or other complications in any patients (including a 90-year-old patient with severe aortic stenosis), marking a complete success.
Seoul St. Mary's Hospital - Case 1
01 Preoperative Assessment
The patient was a 90-year-old male with severe aortic stenosis. CT analysis revealed a moderately calcified trileaflet aortic valve with an annulus circumference of 70.7mm (mean diameter: 22.5mm), LVOT circumference of 68.1mm (mean diameter: 21.7mm), sinotubular junction (STJ) mean diameter of 27.3mm, and ascending aorta mean diameter of 27.4mm. Vascular access was assessed as favorable.
02 Procedure and Outcome
After preoperative discussion, a 24mm valve was selected. The procedure involved pre-dilation with a 16F sheath and deployment using a stiff guidewire, achieving successful implantation.
Seoul St. Mary's Hospital - Case 2
01 Preoperative Assessment
The patient presented with severe aortic stenosis. CT analysis showed a moderately calcified trileaflet aortic valve with an annulus circumference of 77.5mm (mean diameter: 24.7mm), LVOT circumference of 75.4mm (mean diameter: 24.0mm), STJ mean diameter of 27.2mm, and ascending aorta mean diameter of 30.4mm.
02 Procedure and Outcome
A 27mm valve was implanted with Sentinel cerebral protection due to calcification in the aortic arch, resulting in optimal outcomes.
Seoul St. Mary's Hospital - Case 3
01 Preoperative Assessment
The patient was a 70-year-old male with severe aortic stenosis. CT analysis revealed a heavily calcified trileaflet aortic valve with an annulus circumference of 81.2mm (mean diameter: 25.8mm), LVOT circumference of 89.0mm (mean diameter: 28.3mm), STJ mean diameter of 29.2mm, and ascending aorta mean diameter of 37.0mm. While right iliac access was favorable, severe calcification was observed from the external iliac to the aortic arch.
02 Procedure and Outcome
A 30mm valve was selected, and traction-assisted arch crossing was performed to navigate the calcified anatomy, ensuring stable valve placement.
Chonnam National University Hospital - Case 1
01 Preoperative Assessment
The patient was an 81-year-old male with severe aortic stenosis. CT analysis showed a heavily calcified trileaflet aortic valve with an annulus circumference of 78.4mm (mean diameter: 25.0mm), LVOT circumference of 76.1mm (mean diameter: 24.3mm), STJ mean diameter of 29.5mm, and ascending aorta mean diameter of 35.4mm. Right iliac access was assessed as favorable.
02 Procedure and Outcome
After evaluation, a 27mm valve was successfully implanted.
Chonnam National University Hospital - Case 2
01 Preoperative Assessment
The patient was an 88-year-old male with severe aortic stenosis. CT analysis revealed a moderately calcified trileaflet aortic valve with an annulus circumference of 71.4mm (mean diameter: 22.7mm), LVOT circumference of 67.6mm (mean diameter: 21.9mm), STJ mean diameter of 27.2mm, and ascending aorta mean diameter of 36.0mm. Vascular access was favorable.
02 Procedure and Outcome
A 24mm valve was selected and successfully implanted.
Postoperative Evaluation
Following the procedures, Professor Zhang Junjie, along with Dr. Chang Ki Yuk and Dr. Kim Juhan, highly praised the VitaFlow Liberty® system.
Dr. Chang Ki Yuk commented: "Under Professor Zhang Junjie's guidance, the VitaFlow Liberty® demonstrated outstanding clinical performance. Its stable deployment characteristics ensured procedural safety and success."
Dr. Kim Juhan remarked: "The VitaFlow Liberty® was truly impressive. It achieved precise positioning even in highly challenging anatomies while maintaining exceptional stability, significantly boosting our confidence in managing complex cases."
Conclusion
The successful completion of South Korea's first commercial implants marks CardioFlow's official entry into Asia's fourth-largest medical device market, laying a solid foundation for further expansion across the region. Moving forward, the company will continue to advance innovative technologies, delivering high-quality structural heart disease solutions to patients worldwide.















 沪公网安备 31011502014876号
沪公网安备 31011502014876号 are registered trademarks of MicroPort CardioFlow Medtech Corporation.
are registered trademarks of MicroPort CardioFlow Medtech Corporation.