
- Home
- About Us
-
Products
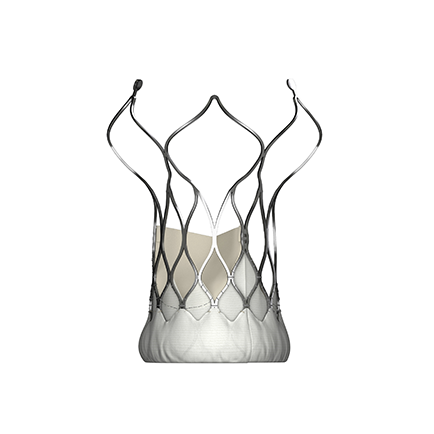
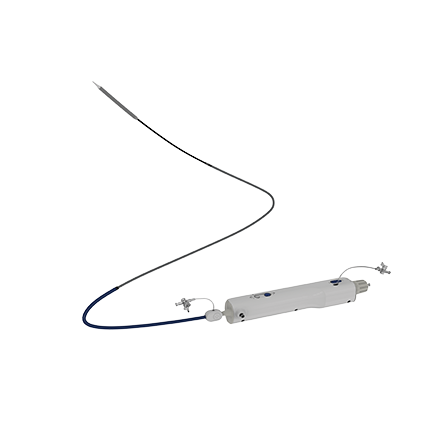
Products Overview VitaFlow® Transcatheter Aortic Valve System VitaFlow Liberty ® Transcatheter Aortic Valve and Retrievable Delivery System Heart Valve Balloon Dilation Catheter Angelguide® Tip-preshaped Super Stiff Guidewire AnchorMan® Left Atrial Appendage Closure System AnchorMan® Left Atrial Appendage Access System
- Clinical Solutions
- Patients
- News
- Investors
- Contact Us












 沪公网安备 31011502014876号
沪公网安备 31011502014876号 are registered trademarks of MicroPort CardioFlow Medtech Corporation.
are registered trademarks of MicroPort CardioFlow Medtech Corporation.